
Did you know that CRISPR technology, particularly Cas9 and sgRNA design, has revolutionized genetic engineering with over 10,000 research papers published in just a few years? It’s pretty mind-blowing! But while we’re all excited about the potential of this groundbreaking tool, it’s crucial to understand its legal and regulatory attributes. Let’s dive into what makes cas9 sgrna design not only innovative but also compliant with various laws.
The Basics of Cas9 sgRNA Design
So, what exactly is Cas9 sgRNA design? In simple terms, it’s a method used to edit genes by guiding the Cas9 enzyme to specific DNA sequences. However, when we talk about its legal aspects, things get interesting. The regulations surrounding gene editing are evolving rapidly as scientists push boundaries. This means that any technical specifications related to safety assessments or ethical considerations must be taken seriously. For instance, countries have different stances on genetically modified organisms (GMOs), which can affect how researchers approach their projects.
Diving into Plasmid Preparation and Technical Specifications
Now let’s chat about plasmid preparation—an essential step in using Cas9 for gene editing. When preparing plasmids containing your desired sgRNAs and other components for delivery into cells, there are strict guidelines you need to follow regarding biosafety levels and containment measures. These technical specifications ensure that any work done does not pose risks to human health or the environment. Additionally, proper documentation during this process is vital for compliance with local regulations; after all, nobody wants an unexpected visit from regulatory authorities!
The Role of Synbio in Technical Specifications
Synthetic biology (Synbio) plays a significant role here too! With advancements in synthetic biology techniques enhancing our ability to create tailored biological systems using tools like CRISPR-Cas9 technology comes new responsibilities under existing laws governing biotechnology products. The technical specifications often require thorough risk assessments before deploying these engineered organisms outside controlled environments—think lab settings versus open fields! Understanding these nuances helps us navigate through complex regulatory frameworks effectively.
Conclusion: Wrapping Up on Technical Specifications for Cas9 sgRNA Design
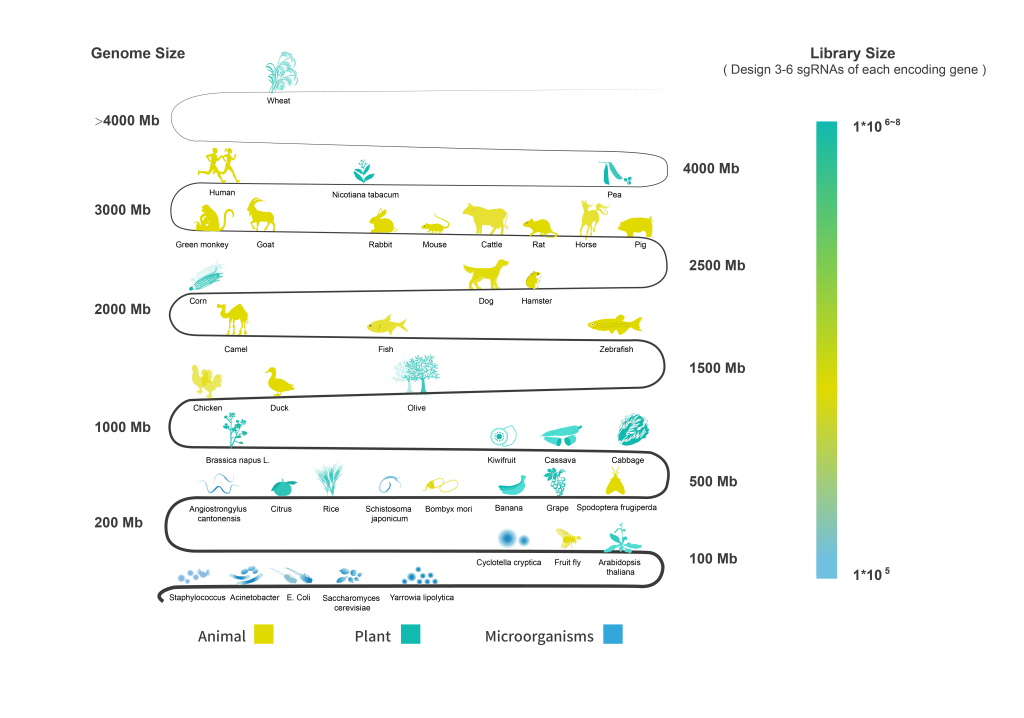
In summary, while exploring the exciting world of Cas9 sgRNA design opens up endless possibilities for genetic innovation; it also brings along a hefty dose of responsibility concerning legal regulations and technical specifications. From ensuring safe plasmid preparations to adhering strictly to Synbio guidelines—being informed keeps us ahead in both science and compliance!
